Aptima® HPV Assays
Identifying the presence and threat of high-risk human papillomavirus (HPV) infection.
Don’t Just Sense a Presence. Sense a Threat
Nearly all sexually active women and men will have an HPV infection at some point in their lives. Very few will go on to develop cancer.1 The Aptima HPV assay targets high-risk HPV mRNA.3 Studies have shown mRNA identifies the presence and activity of a high-risk HPV infection.2,3
The Aptima HPV assay targets the oncogenes E6/E7 mRNA, indicative of the HPV infections most likely to lead to disease.2,3
The Aptima® HPV 16 18/45 Genotype Assay deliver results for type 16, with separate combined result for HPV types 18 and 45, indicating high-risk carcinogenic potential for HPV positive patients.4,5 Identification of these types as part of reflex testing may identify up to 94% of all cervical adenocarcinomas.1
Maximising the Benefits of Screening Programs
With intervals between recommended screenings for cervical cancer extended, identifying patients at risk becomes increasingly important. The Aptima HPV assay, which targets HPV mRNA, has demonstrated the same excellent sensitivity and higher specificity in comparison to DNA based assays to maximise the benefits of cervical cancer screening programmes.6
Proven Performance
Validated for co-testing, HPV primary screening and ASCUS triage, the Aptima HPV Assay has been extensively validated.7 Cross-sectional studies,8-13 longitudinal studies up to 10 years,14-16 and real-life screening program data17 support the performance of the Aptima HPV assay.
Reduce Unnecessary Overtreatment
Improved specificity means minimising false-positive test results, and unnecessary over-treatment for women. It allows clinicians to target the right patients for colposcopy, leading to more efficient screening programs and increased savings. The Aptima HPV Assay showed up to 24% fewer false-positive test results compared to a DNA-based test.18
Scale Automation
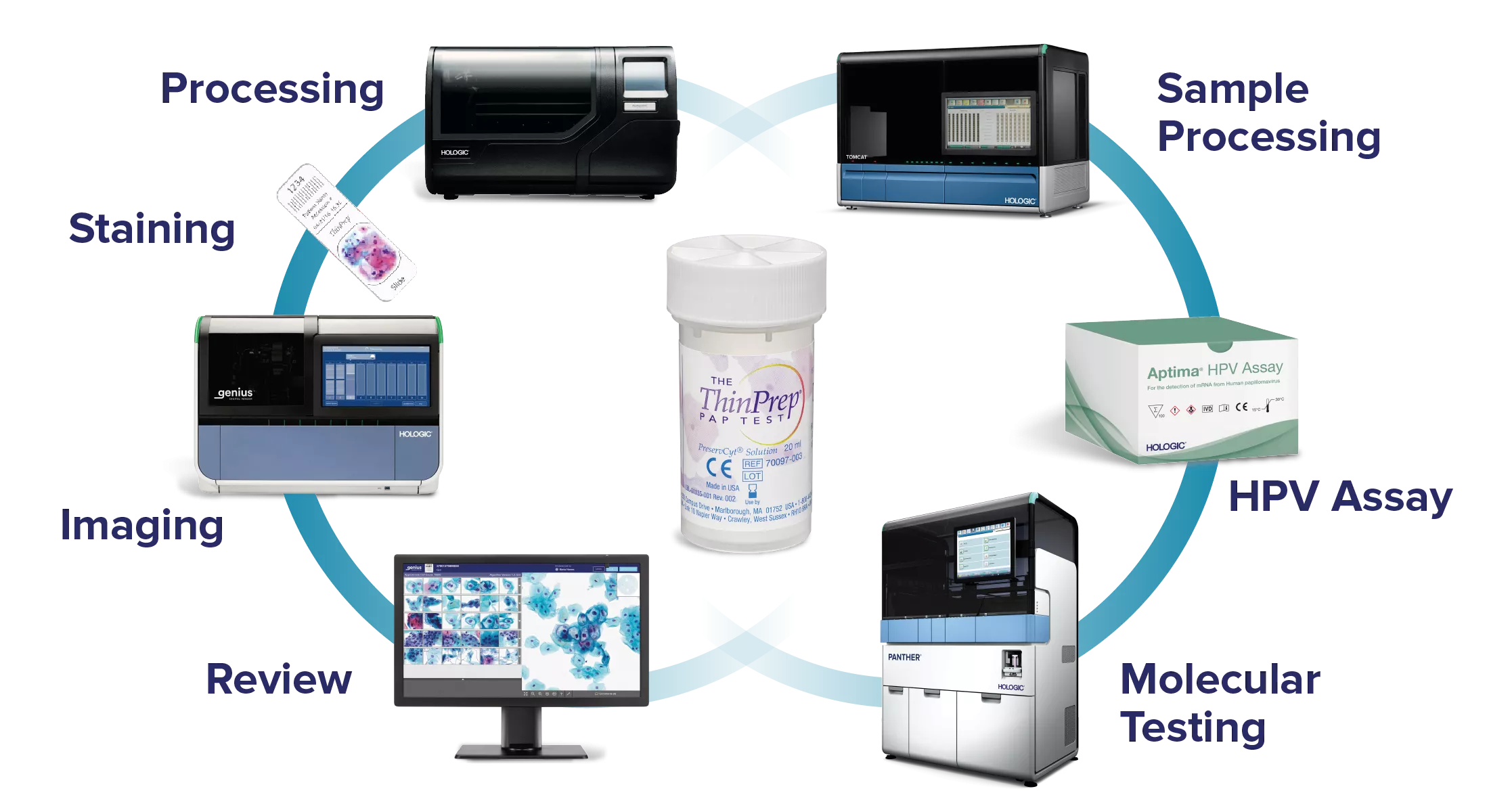
The performance of the HPV assays, combined with a high throughput, fully automated workflow and a complete sample traceability, makes this solution especially relevant for wide screening programmes.19
Working With You to Advance Cervical Health
We pride ourselves on being champions of women’s health and global leaders in screening, dedicated to advancing the accuracy and early detection of cervical cancer. From HPV to cytology, and now also AI-based digital diagnostics, we offer a comprehensive and unique screening portfolio, from sample collection to diagnosis.
Advancing the Early Detection of Cervical Cancer
24% Fewer False-Positive
test results with Aptima HPV assay compared to a DNA-based test18
100+ Million
Aptima HPV tests sold globally20
30 Countries
supporting cervical cancer diagnosis and screening20
100+ Publications
supporting the use of the Aptima HPV assay21

Aptima HPV Assay Design22
- Highly sensitive detection of E6/E7 mRNA oncogenes with isothermal Transcription mediated Amplification (TMA)
- The Aptima HPV assay includes an internal process control as a control of nucleic acid extraction and amplification
- Prevents cross-contamination: Single tube extraction to detection process
- Minimises presence of inhibitors and enhances specificity: Target capture extracts mRNA targets specifically
Aptima HPV Assay Overview
Evidence. Insight. Collaboration.
Our education portal improves patient care through excellence in education, communication of clinical and scientific evidence, and partnerships with the healthcare community.
Genital HPV Infection - CDC Fact Sheet. http://www.cdc.gov/std/hpv/hpv-factsheet-march-2014-press.pdf. Published 2014. Accessed August 24, 2015.
Tinelli A, Leo G, Pisanò M. et al. HPV viral activity by mRNA HPV molecular analysis to screen the transforming infections in precancer cervical lesions. Curr Pharm Biotechnol. 2009 Dec;10(8):767-771.
Cuschieri K, Whitley M, Cubieet H al. Human Papillomavirus Type Specific DNA and RNA Persistence–Implications for Cervical Disease Progression and Monitoring. J Med Virol. 2004 May;73(1):65-70.
Guan P, Howell-Jones R, Li N et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer. 2012 Nov 15;131(10):2349-59.
Tjalma W, Fiander A, Reich O et al. Differences in human papillomavirus type distribution in high-grade cervical intraepithelial neoplasia and invasive cervical cancer in Europe. Int. J. Cancer. 2013;132(4):854–867.
Saslow D, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology Screening Guidelines for the Prevention and early Detection of Cervical Cancer. Am J Clin Pathol. 2012 Apr;137(4):516-42.
Aptima HPV Assay [package insert] AW-22202 Rev. 001. San Diego, CA: Hologic, Inc; 2023.
Szarewski A, Ambroisine L, Cadman L et al. Comparison of predictors for high-grade cervical intraepithelial neoplasia in women with abnormal smears. Cancer Epidemiol Biomarkers Prev. 2008 Nov;17(11):3033-3042.
Monsonego J, Hudgens MG, Zerat L, et al. Evaluation of oncogenic human papillomavirus RNA and DNA tests with liquid-based cytology in primary cervical cancer screening: the FASE study. Intl J Cancer. 2011 Aug;129(3):691-701.
Cuzick J, Cadman L, Mesher D, et al. Comparing the performance of six human papillomavirus tests in a screening population. Br J Cancer. 2013 Mar;108:908-913.
Iftner T, Becker S, Neis KJ, et al., Head-to-Head Comparison of the RNA-Based Aptima Human Papillomavirus (HPV) Assay and the DNA-Based Hybrid Capture 2 HPV Test in a Routine Screening Population of Women Aged 30 to 60 Years in Germany. J Clin Microbiol. 2015 Aug;53(8):2509-16.
Cook D, Smith LW, Law J, et al., Aptima HPV Assay versus Hybrid Capture® 2 HPV test for primary cervical cancer screening in the HPV FOCAL trial. J Clinical Virology 2017 Feb;87:23–29.
Haedicke J. , Iftner T. A review of the clinical performance of the Aptima HPV assay 2016 Mar;76 Suppl 1:S40-S48.
Strang T, Gottschlich A, Cook D et al. Long-term cervical precancer outcomes after a negative DNA- or RNA-based human papillomavirus test result. Am J Obstet Gynecol. 2021 Nov;225(5):511.e1-511.e7.
Iftner et al., Longitudinal Clinical Performance of the RNA-Based Aptima Human Papillomavirus (AHPV) Assay in Comparison to the DNA-Based Hybrid Capture 2 HPV Test in Two Consecutive Screening Rounds with a 6-Year Interval in Germany, J Clin Microbiol. 2019 Jan 2;57(1):e01177-18. doi: 10.1128/JCM.01177-18.
Forslund O et al., HPV-mRNA and HPV-DNA detection in samples taken up to seven years before severe dysplasia of cervix uteri. Int J Cancer. 2019 Mar. 1;144(5):1073-1081. doi: 10.1002/ijc.31819.
Rebolj M, Cuschieri K, Mathews CS, et al. Extension of cervical screening intervals with primary human papillomavirus testing: observational study of English screening pilot data. BMJ 2022; 376:e068776.
Aptima HPV Assay [package insert] AW-22202 Rev. 001. San Diego, CA: Hologic, Inc.; 2023 Table 16
PB-00746-FRA-FR Rév 002 © 2019 Hologic, Inc. PSS Brochure
Data based on Hologic sales numbers since launch in 2012 to 31Jan2020. File reference: AHPVtotalnumberJan2020 (Page 6 https://hologic.box.com/s/w5ccn2nza52n5010pr42ivy4ebfqbgxb)
https://healthdxs.com/en/ View published data
de Sanjose S, Quint WG, Alemany L et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11(11):1048-1056.
Wheeler CM, Hunt WC, Joste NE Ket al. Human papillomavirus genotype distributions: implications for vaccination and cancer screening in the United States. J Natl Cancer Inst. 2009 Apr 1;101(7):475-87.
Coutlée F, Ratnam S, Ramanakumar AV et al. Distribution of human papillomavirus genotypes in cervical intraepithelial neoplasia and invasive cervical cancer in Canada. J Med Virol. 2011 Jun;83(6):1034-41.
Hopenhayn C, Christian A, Christian WJ et al. Prevalence of human papillomavirus types in invasive cervical cancers from 7 US cancer registries before vaccine introduction. J Low Genit Tract Dis. 2014 Apr;18(2):182-9.
Safety Data Sheets
Package Inserts
Related Products
1434
2797
Hologic BV, Da Vincilaan 5, 1930 Zaventem, Belgium.
Notified Body number wherever applicable